Biopharmaceutical chromatography system
Key words:
Classification:
Biological drug purification and separation equipment
- Product Description
- Technical parameters
- More introduction
-
- Commodity name: Biopharmaceutical chromatography system
- Commodity ID: CXTH23-018
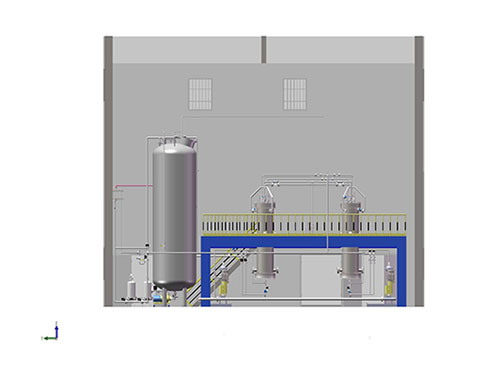
System Introduction:
The system is used in the context of biotechnological drug purification. The system design focuses on the inactivation of macromolecular biological samples, the establishment of low shear fluid transport, the use of biological compatible contact materials, widely used in ion exchange and gel filtration chromatography and other purification processes.
The software system designed according to the certification requirements of pharmaceutical companies fully meets the requirements of FDA21CFRpart11 and GAMP5 regulations, and is widely used in the production of proteins, vaccines and nucleic acid drugs.
The system can be customized according to the needs of users, such as infusion unit can be customized according to the actual flow rate, pressure; can also be customized to the detection unit, according to the needs of UV, PH, conductivity and other detection means.
Core technology:
■Automatic On-line Bubble Removal Technology
■Membrane Emulsification Preparation Medium Technology
■chromatographic column partitioning technique
■Anti-interference Technology of Integrated Multi-dimensional Detector
■Precision Shunt Technology of Multi-channel Detection Interface
■Intelligent Software Cloud Processing Technology