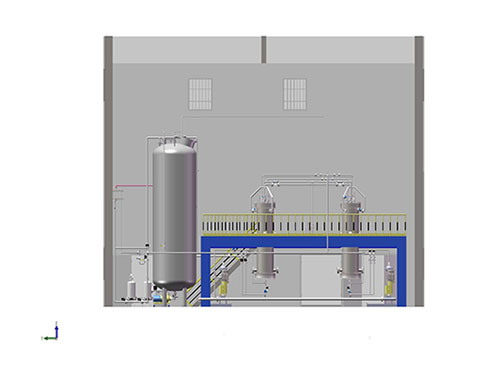
AIC-101 impurity separation and purification system
Key words:
Classification:
Intelligent impurity capture instrument
- Product Description
- Technical parameters
- More introduction
-
- Commodity name: AIC-101 impurity separation and purification system
- Commodity ID: CXTH23-019
Even trace levels of impurities can affect drug quality and patient safety.
The impurities even at trace levels can impact drug quality and patient safety.
Impurities in drugs and drug products should be isolated and characterized as long as their content reaches the threshold level specified by ICH.
Impurities in drug substance and drug product present at threshold levels recommended by the International Conference on Harmonization (ICH) should be isolated and characterized.
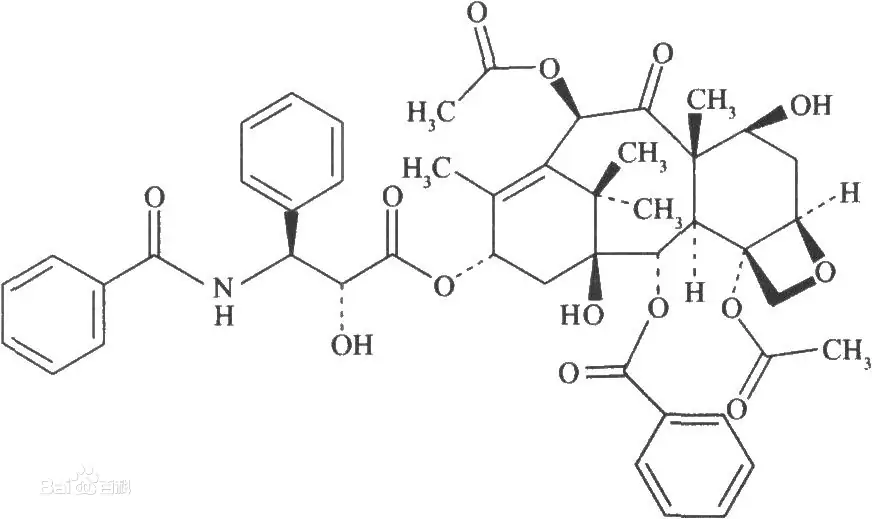
The physical and chemical properties of low concentration impurities are close to those of active pharmaceutical ingredients, which are hidden under the main peak, and it is difficult to obtain complete baseline separation in a single chromatographic operation.
These low-concentrated impurities hidden below a main peak have physicochemical properties close to the active drug substance; thereby a single chromatographic run may be insufficient to achieve full baseline separation.
the importance of impurity research:
✍Ensuring the safety and effectiveness of drugs is a basic principle to be followed in drug development and drug evaluation;
✍The stability and control of drug quality is the premise and foundation to ensure the safety and effectiveness of drugs;
✍Impurity research is an important part of drug quality research.
System features:
1.High processing efficiency (especially suitable for 10-100mg sample requirements);
2.Impurities are captured and accumulated in the system, which can obtain high-concentration impurities, which is conducive to subsequent evaporation and recovery;
3.Applicable to a wide range, can capture the pre-miscellaneous and post-miscellaneous, but also from the natural extract in the directional capture of related components;
4.The separation degree of the target impurity and the main component is not required to be high, only the mobile phase composition is sensitive to the influence of the impurity or the main component retention.